Background: The ATHNdataset is sponsored by the American Thrombosis and Hemostasis Network, including 17,109 hemophilia A people as of the cutoff date, 4/30/22.
Patients with Hemophilia A are transitioning more and more from standard half-life (SHL) products to extended half-life (EHL) recombinant factor VIII (rFVIII) products
Objective: To evaluate the effect of Hemophilia A patients transitioning from BAY 81-8973 (Kovaltry ®) to BAY 94-9027 (Jivi ®) and from BAY 14-2222 (Kogenate FS ®) to BAY 81-8973 and then to BAY 94-9027 in a real-world setting.
Methods: The ATHNdataset was queried for patients treated with BAY 94-9027 that had BAY 81-8973 as a prior medication as well as for the latter that had BAY 14-2222 as a prior medication. Data included demographic data, treatment history, and bleed rates. Query dates were between January 1, 2010 and April 30, 2022.
Summary: A total of 205 patients were treating with BAY 94-9027 and 354 with BAY 81-8973 at data cut-off.
Thirty patients that treated with BAY 94-9027 had BAY 81-8973 listed as one of their prior medications. Of these, 90% had severe Hemophilia A, while 7% had moderate and 3% had mild disease. All were male, with an average age of 37 years at data cut-off; 80% being White and 90% not Hispanic, Latino or of Spanish origin.
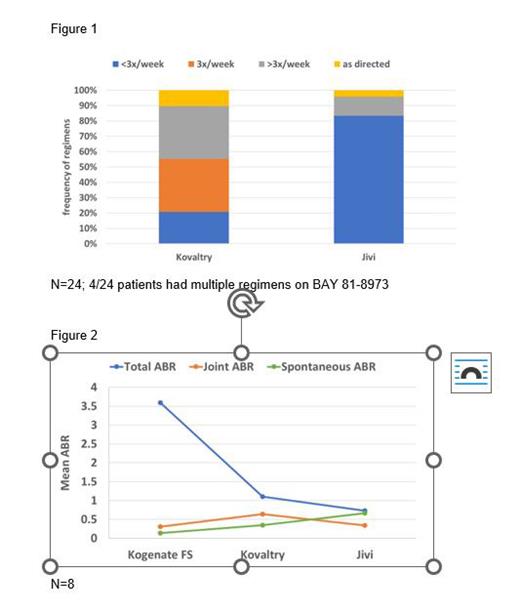
Overall, 24/30 patients were consistently treated prophylactically with BAY 81-8973 and with BAY 94-9027. The mean annualized total bleed rate (Total ABR) on BAY 81-8973 was 0.47 and 0.42 on BAY 94-9027, while the annualized joint bleed rate (Joint ABR) was 0.28 on both products and the annualized spontaneous bleed rate (Spontaneous ABR) was 0.17 and 0.30, respectively. The most frequent dosing regimen on BAY 81-8973 was 3x/week or more often, while on BAY 94-9027 more than 80% of patients were using a less frequent regimen than 3x/week, with 2x/week being the most common. (Figure 1)
In addition, a sub-analysis was performed on the same patients that transitioned from BAY 81-8973 to BAY 94-9027, to include those patients that also used BAY 14-2222 as a prior medication to BAY 81-8973. A total of 12 patients transitioned from BAY 14-2222 to BAY 81-8973 and then to BAY 94-9027. Of these, all (100%) had severe Hemophilia A and were male. The average age was 33 years at data cut-off; 83% being White and 92% not Hispanic, Latino or of Spanish origin.
Overall, 8/12 patients were treating consistently prophylactically with BAY 14-2222, BAY 81-8973 and BAY 94-9027. For these patients, the mean Total ABR on BAY 14-2222 was 3.59, on BAY 81-8973 it was 1.10 and 0.72 on BAY 94-9027, while the Joint ABR was 0.30 on BAY 14-2222, 0.64 on BAY 81-8973 and 0.34 on BAY 94-9027. The Spontaneous ABR was 0.14, 0.35 and 0.67, respectively. (Figure 2) The most frequent dosing regimen on BAY 14-2222 and BAY 81-8973 was 3x/week, while on BAY 94-9027 nearly 90% of patients were using a less frequent regimen than 3x/week, with 2x/week being the most common.
Conclusions: The data show that hemophilia A patients who transitioned to BAY 94-9027 from BAY 81-8973, or those that transitioned from BAY 14-2222 to BAY 81-8973 and then to BAY 94-9027 did experience similar or decreased total annual bleed rates with a decrease in their prophylaxis regimen frequency when transitioning to BAY 94-9027 in the real world. The therapeutic burden of frequent infusions can be reduced when patients transition between rFVIII product classes (SHL to EHL), without change in their annual bleed rate.
These data should be interpreted with caution owing to limitations of real-world studies and further studies are needed to confirm the impact of switching to BAY 94-9027 and BAY 81-8973 in real-world settings.
Additional studies are needed to expand on these results.
Disclosures
Charlet:Bayer US LLC Pharmaceuticals: Current Employment. Moulton:Bayer US LLC Pharmaceuticals: Current Employment. Recht:NovoNordisk: Research Funding; BioMarin: Research Funding; ATHN: Membership on an entity's Board of Directors or advisory committees; Grifols: Research Funding; uniQure: Consultancy, Research Funding; Bayer Pharmaceuticals: Research Funding; LFB: Research Funding; Partners in Bleeding Disorders: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Pfizer: Consultancy; Hema Biologics: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal